Previous product spotlights have highlighted the PD-1 pathway; however, there are many other immune checkpoint pathways involved in T cell activation and anti-tumor responses. Researchers are investigating several of these targets as combinational therapies along with PD-1 inhibitors. One pathway that has been of particular interest is the TIGIT/CD226 pathway, because it acts by a novel mechanism to regulate CD8+ T cell functions within the tumor microenvironment.
The TIGIT/CD226 pathway parallels the CD28/CTLA-4 pathway that we discussed in previous newsletters. Similar to CD28 and CTLA-4, CD226 and TIGIT share ligands, CD155 (also known as the poliovirus receptor, PVR) and CD112 (Nectin-2). CD226 and TIGIT (shown here as VSTM3) can compete for ligand binding on target cells. However, CD226 and TIGIT binding have opposite results: while engagement of CD226 with CD155 or CD112 enhances T cell activation, the interaction between TIGIT and CD155 or CD112 inhibits T cell responses.
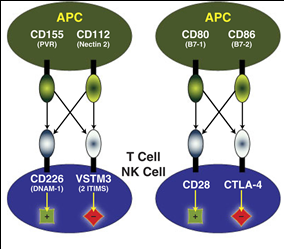
Both CD155 and CD112 are found on dendritic cells (DC) and macrophages, and both are highly overexpressed on multiple cancer cell lines and primary tumors. CD226 (also called DNAM-1) is expressed primarily on NK cells and CD8+ T cells. The CD226 glycoprotein acts as a co-stimulatory adhesion molecule, promoting Th1 differentiation, and triggering NK activation. Interactions between CD226 and the CD155/CD112 ligands play an important role in T cell and natural killer (NK) cell–mediated recognition and lysis of tumor cells.
On the other hand, CD155/CD112 binding to TIGIT (T-cell immunoreceptor with immunoglobulin and ITIM domains) directly suppresses the cytolytic activity of NK cells and inhibits T cell proliferation and cytokine production in CD4+ T cells. TIGIT also indirectly inhibits T cell responses by triggering CD155 in DCs, thereby preventing DC maturation and inducing production of immunosuppressive cytokines such as IL-10. Additionally, TIGIT has been shown to inhibit proinflammatory Th1 and Th17 responses. TIGIT can even physically block the dimerization of CD226, which prevents its costimulatory function.

Not surprisingly, researchers are actively developing therapeutic monoclonal antibodies to regulate these pathways. Neutralizing the TIGIT pathway is especially promising because it blocks the negative TIGIT inhibitory signal on effector T cells while simultaneously promoting the positive CD226 stimulatory signals for T cell proliferation and proinflammatory cytokine production [IFN-γ, IL-17, IL-9], leading to T cell activation. Antibodies that block TIGIT binding to CD155 have been shown to prevent TIGIT’s down-regulation of NK cytotoxicity , and to decrease tumor growth. Similarly, an anti-CD112 monoclonal has been show to exert an in vivo anti-tumor effect on breast and ovarian cancer cells, which suggests the potential of CD112 as a target for antibody therapy for cancer treatment.
TIGIT expression is strongly associated with expression of other co-inhibitory molecules; PD-1, Tim-3 and Lag-3. Thus many researchers are investigating combinations of blocking antibodies. Antibody blockade of the TIGIT pathway synergizes with Tim-3 blockade to maximally decrease tumor growth. Likewise, blockade of TIGIT signaling has been shown to synergize with PD-1/PD-L1 blockade to enhance antitumor CD8+ T cell responses in both humans and mice (see Cancer Cell figure above). In particular, antibody co-blockade of TIGIT and PD-L1 results in significant tumor clearance, and elicits tumor rejection in preclinical models.
In addition to cancer, the CD226/TIGIT pathway has been linked to several autoimmune and inflammatory diseases. For example, TIGIT engagement has been shown to ameliorate collagen-induced arthritis, while down-regulation of TIGIT exacerbates experimental autoimmune encephalomyelitis. CD226 binding to CD155 has been associated with autoimmune-linked disorders, including multiple sclerosis and type 1 diabetes, and treatment with a neutralizing anti-CD226 monoclonal efficiently inhibits activation and proliferation of T cells from patients with autoimmune diseases. Even better, naive T cells do not express CD226, so therapeutic strategies targeting CD226 would exclusively target proinflammatory Th1 and Th17 cells.
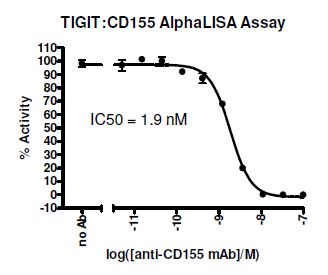
Overall, targeting the CD226/TIGIT pathway may provide a therapeutic approach that can modulate the pro-inflammatory and anti-inflammatory balance in a wide range of diseases.
BPS provides 4 unique kits for investigating the CD226/TIGIT pathway. These homogeneous kits allow screening for the identification of small molecule inhibitors and antibodies that block receptor-ligand interaction using AlphaScreen technology. BPS also offers the individual biotinylated and unlabeled proteins,
